Abstract
Introduction: Concizumab is an anti-tissue factor pathway inhibitor (TFPI) monoclonal antibody, currently in clinical phase 3 trials as a once-daily subcutaneous prophylaxis for all hemophilia subtypes. Physical activity was historically discouraged in patients with hemophilia, but recent studies have reported improvements in proprioception, muscle strength and joint health with appropriate physical activities. Here we report, for the first time, results of an exploratory analysis on physical activity from the concizumab explorer7 study (NCT04083781) in patients with hemophilia A or B with inhibitors (HAwI/HBwI).
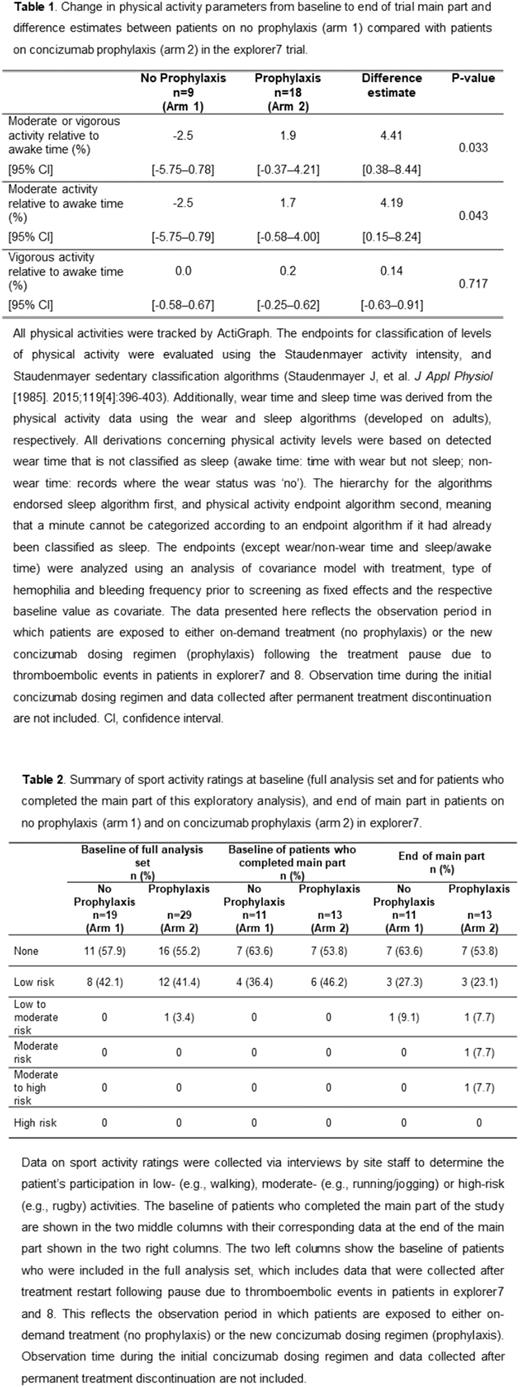
Methods: In explorer7, patients from outside the concizumab program, as well as patients previously treated on-demand in explorer6 (NCT03741881; non-interventional study), were recruited and randomized 1:2 to no prophylaxis, who continued their on-demand treatment with a bypassing agent as usual (arm 1), or concizumab prophylaxis (arm 2).Physical activity data were collected using the ActiGraph tracker, which was worn on the patients’ non-dominant wrist. Patients were instructed to wear the tracker for two consecutive weeks prior to concizumab treatment start, if not already collected in explorer6 (baseline values) and for eight consecutive weeks at the end of the main part (≥32 weeks on concizumab prophylaxis). Analysis of covariance with treatment, type of hemophilia and bleeding frequency prior to screening as fixed effects and the respective baseline value as covariate, was used to compare the change in physical activity parameters from baseline to end of main part between groups. The patients were also interviewed about sport activity by site staff prior to concizumab treatment (baseline) and at the end of the main part to determine the patient's participation in low- (e.g., walking), moderate- (e.g., running/jogging) or high-risk (e.g., rugby) activities.
Results: A total of 52 male patients with HAwI/HBwI were randomized to no prophylaxis (arm 1; n=19) and concizumab prophylaxis (arm 2; n=33). In total, 25 randomized patients were not included in this analysis (n=10 from arm 1 and n=15 from arm 2); 4 patients did not restart treatment following pause due to thromboembolic events in patients in explorer7 and 8, 6 patients were withdrawn, and 15 patients did not have data from baseline and/or the end of the main part of the study (e.g., broken device, device not being able to transfer data, patients forgetting to wear the device). Patients on concizumab prophylaxis had an increased percentage of awake time spent in moderate physical activity, and moderate-to-vigorous physical activity compared with patients on no prophylaxis (Table 1), with an estimated difference of 4.19% (95% confidence interval [CI]: 0.15-8.24; P=0.043) and 4.41% (95% CI: 0.38-8.44; P=0.033), respectively. For example, if the mean awake time was 12 hours, this translates to 30 minutes of moderate, or moderate-to-vigorous physical activity in patients on concizumab prophylaxis. Mean levels of physical activity relative to awake time were similar at baseline for both groups. Results of the sport activity ratings for patients who completed the main part of the study showed that at baseline, no patients reported participating in low-to-moderate, moderate, moderate-to-high or high-risk sports (Table 2). At the end of main part, patients participated in low-to-moderate-risk (1 patient; 7.7%), moderate-risk (1 patient; 7.7%) and moderate-to-high-risk (1 patient; 7.7%) sports in the concizumab prophylaxis group, and one patient (9.1%) participated in low-to-moderate-risk sports in the no prophylaxis group.
Conclusion: Increased percentage awake time spent in moderate, or moderate-to-vigorous physical activity was observed in patients on once-daily, subcutaneous concizumab prophylaxis, with a significant estimated difference compared with patients with no prophylaxis. While the results should be interpreted with caution due to the smaller cohort of patients included in the current exploratory analysis, prophylactic treatments such as concizumab could encourage patients to spend more time engaging in physical activity that is also of appropriate (higher) risk, which could in turn improve their muscle strength and joint health. Changes in physical activity were also investigated in hemophilia patients without inhibitors in explorer8 phase 3 study.
Disclosures
Villarreal Martinez:Novo Nordisk: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Roche: Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; CSL-Behring: Honoraria, Speakers Bureau; Bayer: Honoraria, Speakers Bureau; Grifols: Honoraria, Speakers Bureau; Octapharma: Honoraria, Speakers Bureau. Cepo:Novo Nordisk: Current Employment, Current equity holder in private company. Laursen:Novo Nordisk: Current Employment; Falck: Ended employment in the past 24 months. Odgaard-Jensen:Novo Nordisk: Current Employment. You:Jugai pharma: Research Funding; Sanofi: Research Funding; Novo Nordisk: Research Funding. Windyga:Alnylam: Research Funding; Bayer AG: Honoraria; CSL Behring: Honoraria; Novo Nordisk: Honoraria, Research Funding; Octapharma: Honoraria; Roche: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Sobi: Honoraria; Swixx BioPharma: Honoraria; Takeda: Honoraria, Research Funding; Novartis: Honoraria; Amgen: Honoraria; Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal